SyphilisStreamline syphilis testing on a platform built for high-powered automation
High global rates of syphilis1 require high-volume testing capabilities across multiple lab settings2. As laboratories strive to keep pace with the growing sexually transmitted infections (STIs) testing demands, the decision-making process for selecting a syphilis assay provider is increasingly influenced by factors such as assay consolidation, confidence in clinical results, and overall cost efficiency.
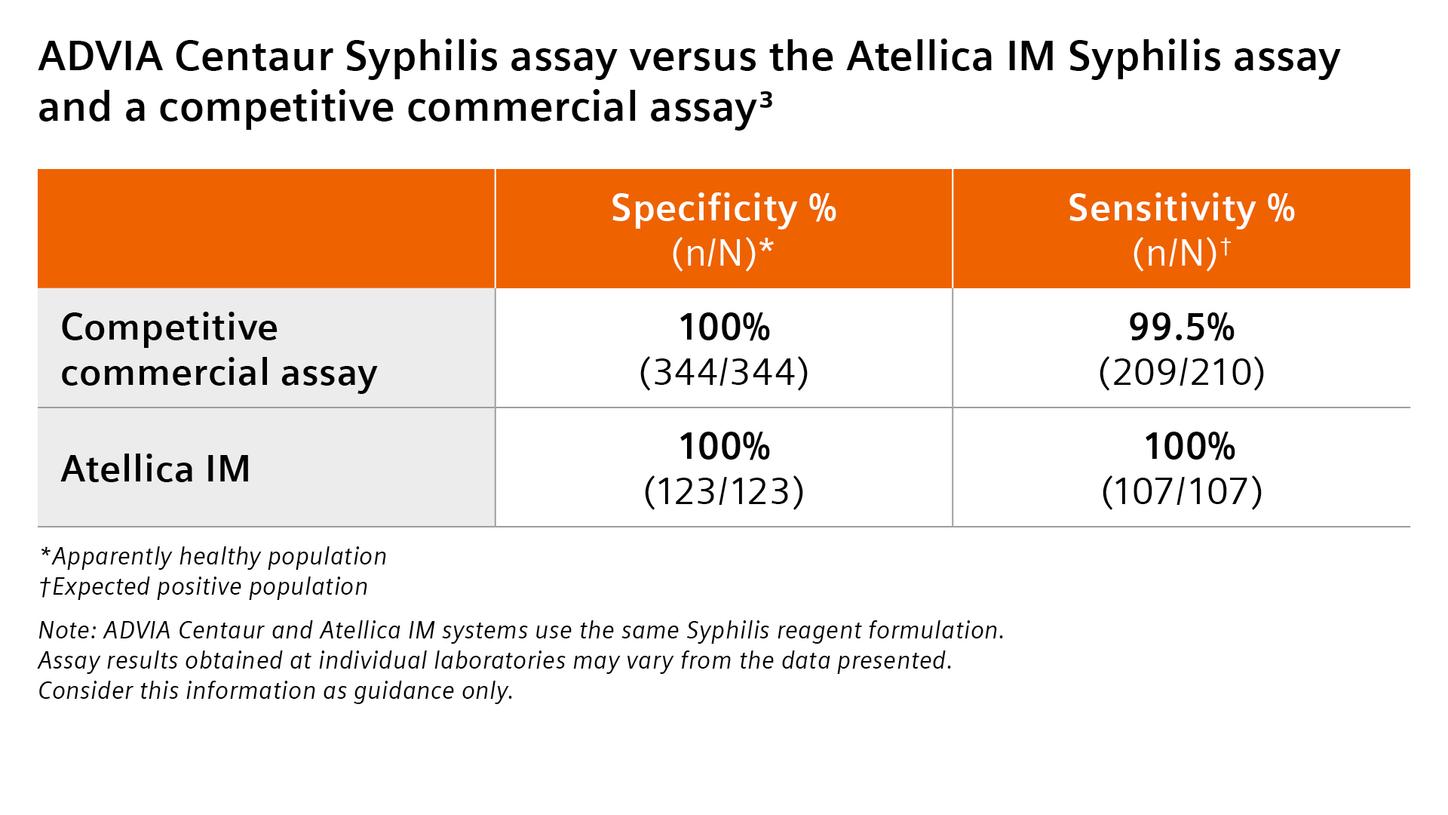
Traditional syphilis testing places significant demands on already overburdened staff. Addressing these challenges, the Atellica IM Syphilis assay, with its high sensitivity and specificity3, delivers a streamlined treponemal testing solution that enhances workflow efficiency to complement the syphilis testing algorithm.
Benefits
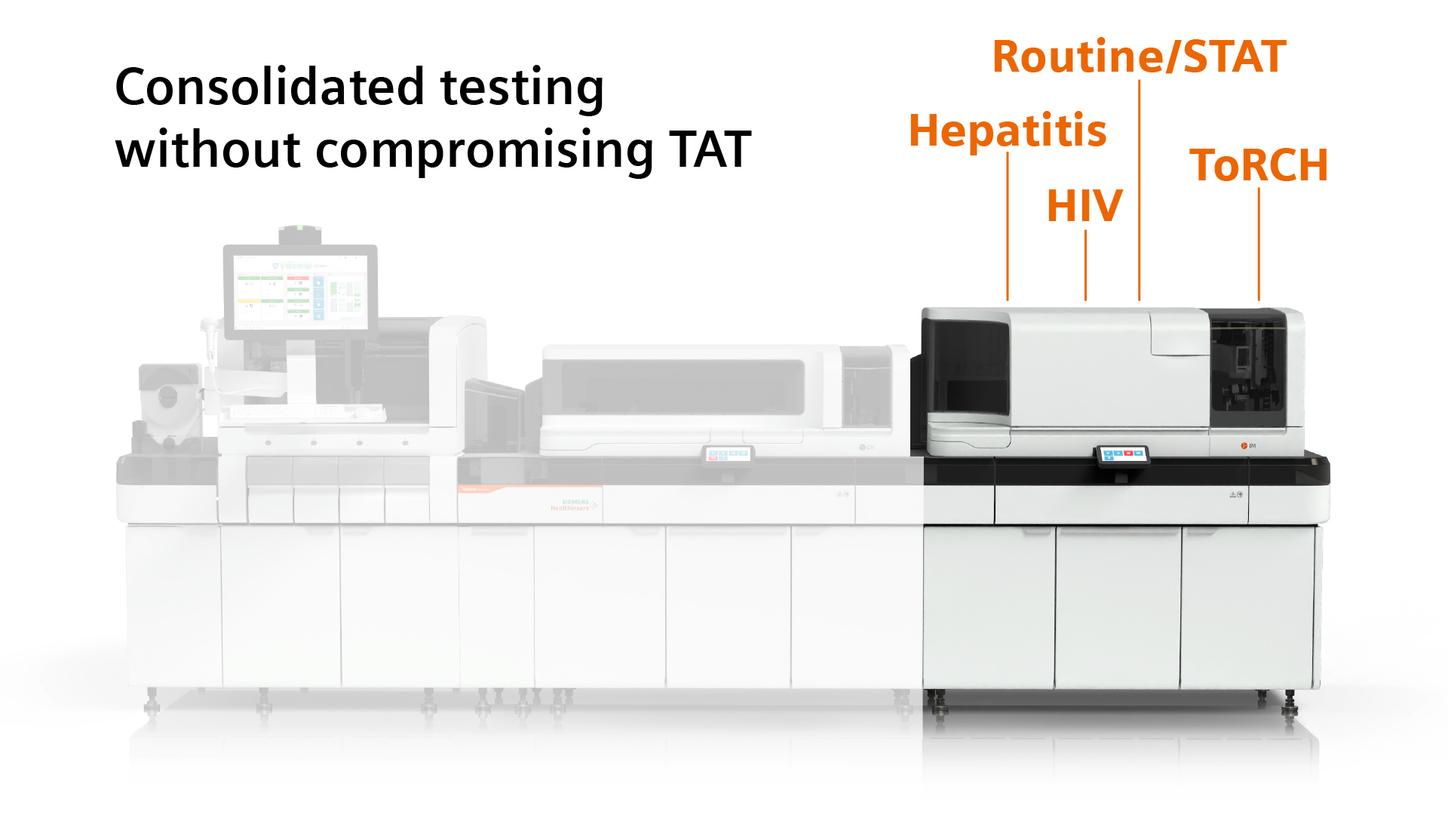
For even greater operational impact, laboratories can consolidate their infectious disease testing on Atellica immunoassay analyzers, engineered for high-performance assays and powered with built-in automation. This smart consolidation approach boosts throughput, reduces manual touchpoints, minimizes opportunities for error, and helps protect staff from biohazards. Elevate control, maximize efficiency, and achieve better results for your lab with a more intelligent, integrated approach to infectious disease testing.
Achieve reliable, accurate detection with the Atellica IM Syphilis Assay, a treponemal assay with high sensitivity and specificity,3 designed to detect antibodies (including IgM and IgG )12 to Treponema pallidum.3 Deliver the clinical confidence your team depends on with performance designed to support precise, timely decision-making.
The Atellica IM Syphilis Assay is powered by Siemens Healthineers' exclusive Acridinium Ester (AE) derivatives. This technology elevates assay performance, delivering high sensitivity and specificity for confident results.
Designed with sustainability in mind, the assay delivers meaningful operational efficiencies through a high number of tests per pack, long calibration intervals and strong onboard stability. This results in fewer consumables, reduced waste potential, and a smart resource-efficient workflow. Choose a solution designed to deliver high performance while supporting your labās commitment to sustainability.
Consolidate syphilis testing alongside hepatitis, HIV, ToRCH, and a growing ID menu, as well as routine and STAT assays on the Atellica Solution without compromising turnaround time4. This high-capacity, fully automated system is designed to elevate productivity, reduce send-outs, and support timely, confident clinical decisions.
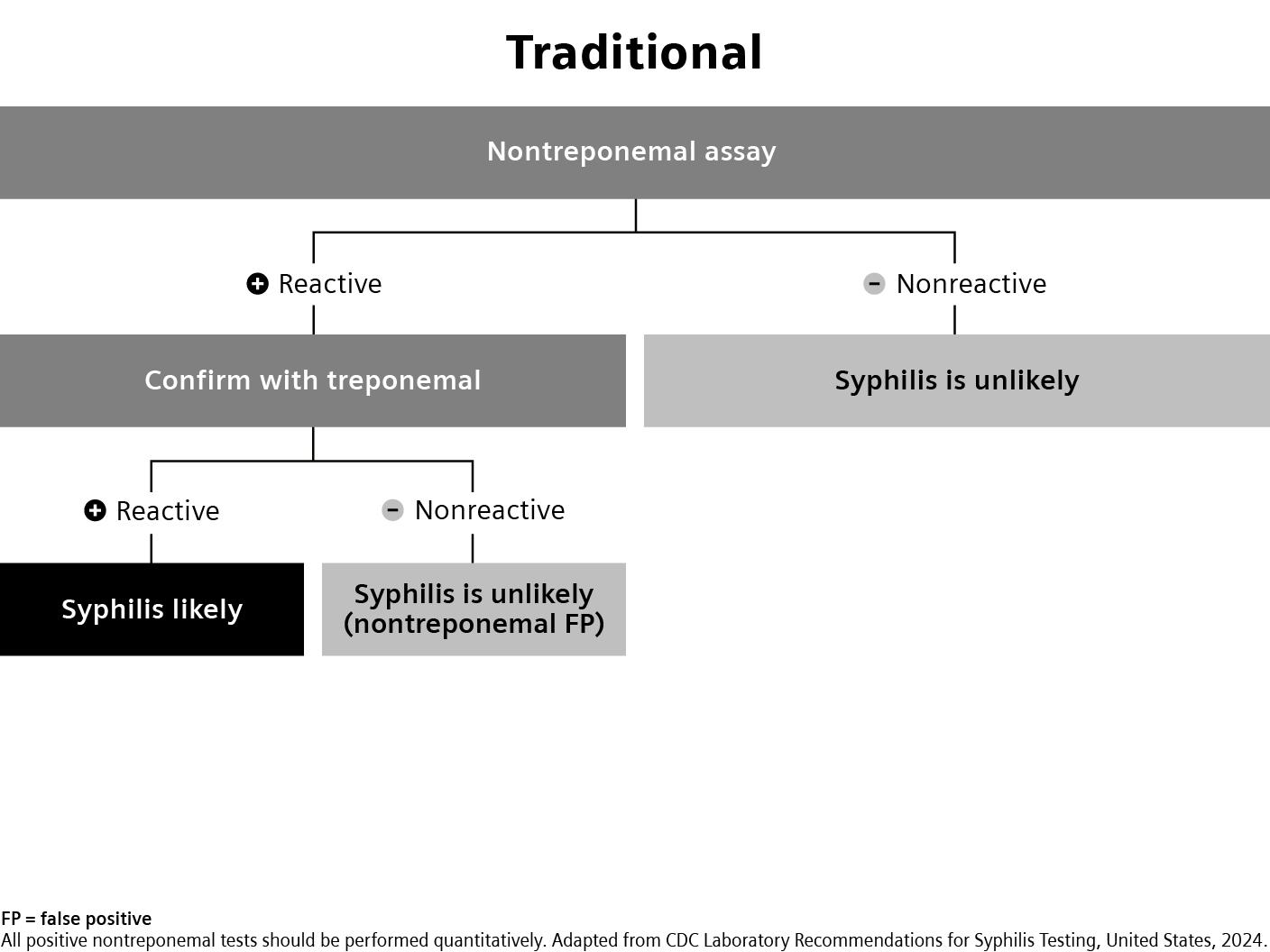
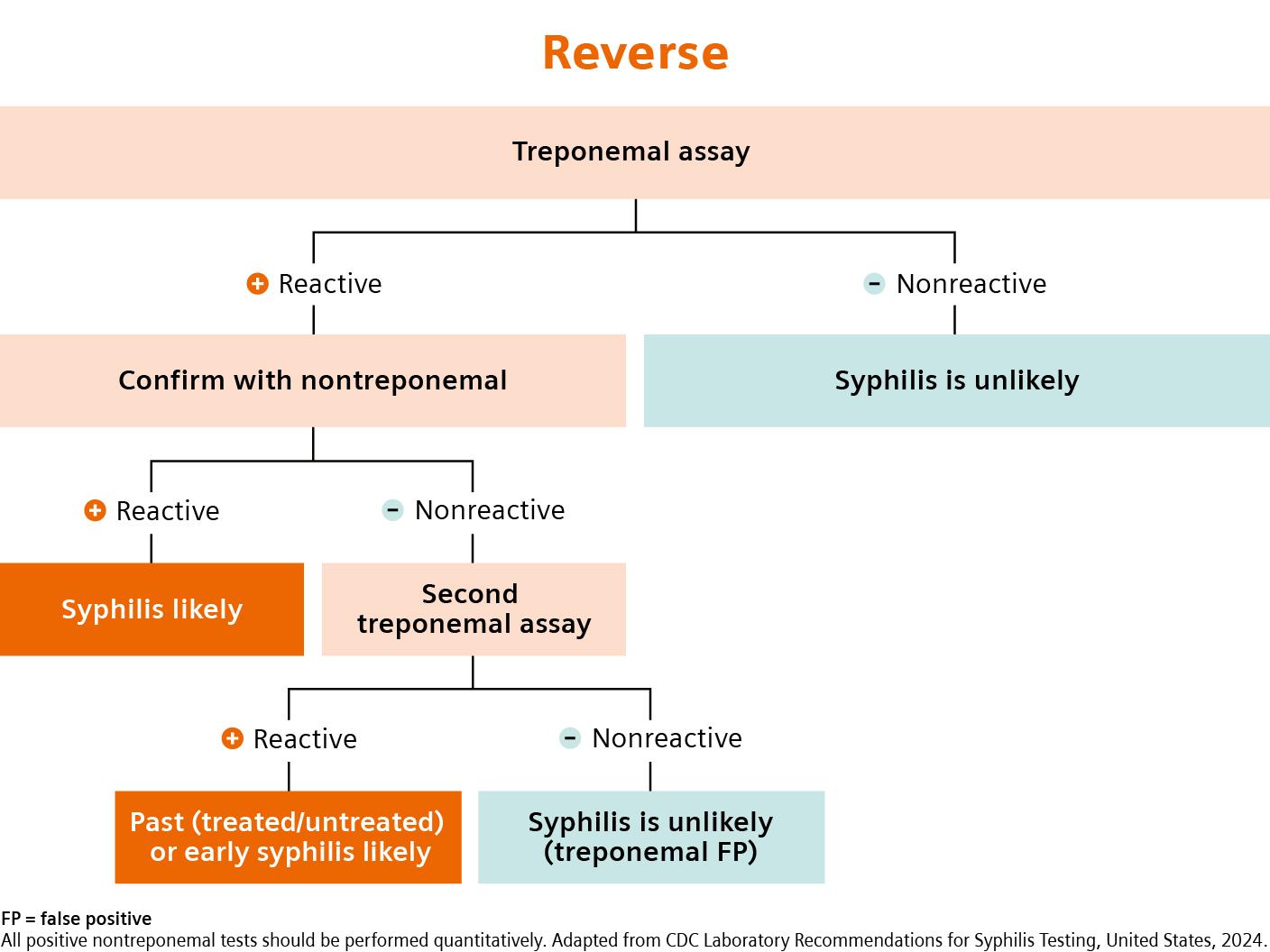
Latest Syphilis testing guidelines
Technical Details
Assay Specifications
Clinical utility
The Atellica IM Syphilis assay aids in the diagnosis of syphilis and is used for the qualitative determination of total (including IgG and IgM) Treponema pallidum antibodies. The assay uses recombinant Tp15 and Tp17 antigens.
Sample type
Serum, plasma (EDTA, lithium heparin, sodium heparin, citrate)
Time to first result
29 minutes
Number of tests per pack
200